
The Covid-19 pandemic reminds all stakeholders that the fulfillment of the need for Medical Devices (Alkes) in Indonesia still depends on imported medical devices. Responding to the Government's commitment and the current needs of society, ASKI (PT Astra Component Indonesia), supported by BIM (PT Berkah Installation Medika) as a distributor with the trademark "SIGOGRIN" launched High Flow Nasal Canula (HFNC). HFNC is a high-flow oxygen therapy device, to assist breathing by distributing oxygen through a clear, transparent and flexible tube. Its use is not limited to Covid-19 patients, but can also be used for patients who have a diagnosis of chronic obstructive pulmonary disease, Restrictive Thoracic Diseases (RTD), Obesity Hypoventilation Syndrome 5, chest wall deformity, neuromuscular disease, and Decompensated Obstructive Sleep Apnea.
The launch of the breathing apparatus, which was developed with PT Astra Otoparts Tbk – Engineering Development Center Division, was symbolically carried out by ASKI President Director Prihatanto Agung Lesmono, BIM President Director Boge Ronny, and BIM founder Lissa Imelia at ASKI, Citeureup, Bogor. "With the cooperation that has been established with BIM, in the future we hope to be able to market other medical devices along with their licensing gradually, in an effort to meet the needs of the community, so that it can run well," said Prihatanto.
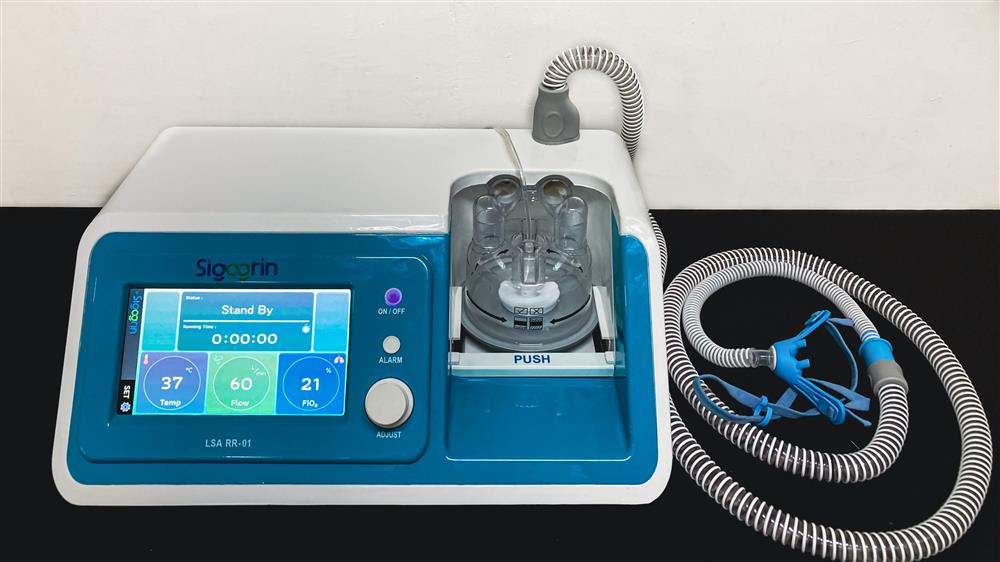
HFNC products can be obtained by the public through BIM as the sole distributor. In order to complement the quality improvement and community satisfaction, BIM will also open a service center to serve the community's needs for the availability of spare parts or spare parts from domestic medical equipment. "Of course we will provide good after-sales service and integrated with calibration services through optimizing cooperation with Alfakes (Association of Indonesian Health Facility Testing and Calibration Laboratories) which will help ensure the feasibility and safety of medical devices," added Boge Ronny. The collaboration between ASKI and BIM, which prioritizes patient safety guarantees on every medical device it produces, should be appreciated so that local medical devices will be able to compete with imported medical devices both in terms of quality and safety.
The government, through the Coordinating Ministry for Maritime Affairs and Investment of the Republic of Indonesia, responds to the condition of dependence on imports by encouraging the acceleration of the development of the domestic medical device industry by carrying out Seven Strategic Steps to Increase Market Availability for Domestic Medical Devices which consist of; (1) Supporting PDN through government spending on goods or services, (2) Increasing domestic medical equipment production capacity, (3) Subsidy for TKDN certification through PEN funds, (4) Incentive schemes for medical equipment and pharmaceutical investors, (5) Improvement of medical equipment with technology research-based, (6) Deadline policy for purchasing imported products, (7) Priority for displaying PDN in E-Catalog.